Welcome to the Future of Drug Development, Where Data-Driven Modeling Meets Real-World Impact
Model-Informed Drug Development is transforming how therapies are designed, tested, and delivered. By embedding quantitative modeling and simulation into every stage of the development lifecycle, MIDD empowers teams to make smarter, faster, and more cost-effective decisions.
At the forefront of this transformation are industry leaders who are redefining what's possible:
- Johnson & Johnson is rethinking Maximum Tolerated Dose (MTD) strategies to accelerate timelines and reduce patient burden.
- Pfizer is using MIDD to define rational starting doses for complex biologics, improving safety and precision from the outset.
- Eli Lilly is leveraging model-based strategies to select optimal drug candidates and make go/no-go decisions earlier, saving time, resources, and lives.
Attendees explored case studies across oncology, CNS, and immunology that demonstrate MIDD’s power from early candidate selection to late-phase dosing. Whether you're refining trial design or preparing for regulatory submission, this summit was your one-stop hub for unlocking the full potential of model-informed strategies.

What you Missed:
Decode the M15 Draft with Exclusive Regulator Insights
Hear firsthand from the experts shaping the latest draft ICH M15 guidelines, and leave with actionable clarity on what’s changing, why it matters, and how to stay ahead in a rapidly evolving regulatory landscape.
Hear Real-World Regulatory Success Stories
Go beyond theory with powerful, real-world examples of how model-informed strategies have secured approvals, so you can shape submissions that stand out and accelerate your path to market.
Advance Your Trial Design with MIDD from Day 1
Discover how leading teams are embedding MIDD at the core of their strategic planning, driving smarter decision-making, faster timelines and greater confidence in your data.
See the Impact of MIDD Across Various Therapeutic Areas
Dive into compelling case studies spanning oncology, CVRM, RNA, Immunology, CNS and beyond to understand where MIDD is transforming development and delivering measurable results.
Bridge the Communication Gap Between Modelers & Biologists
Gain practical strategies to align and build cross functional teams, making MIDD implementation seamless, collaborative and a driving force for innovation across your pipeline.
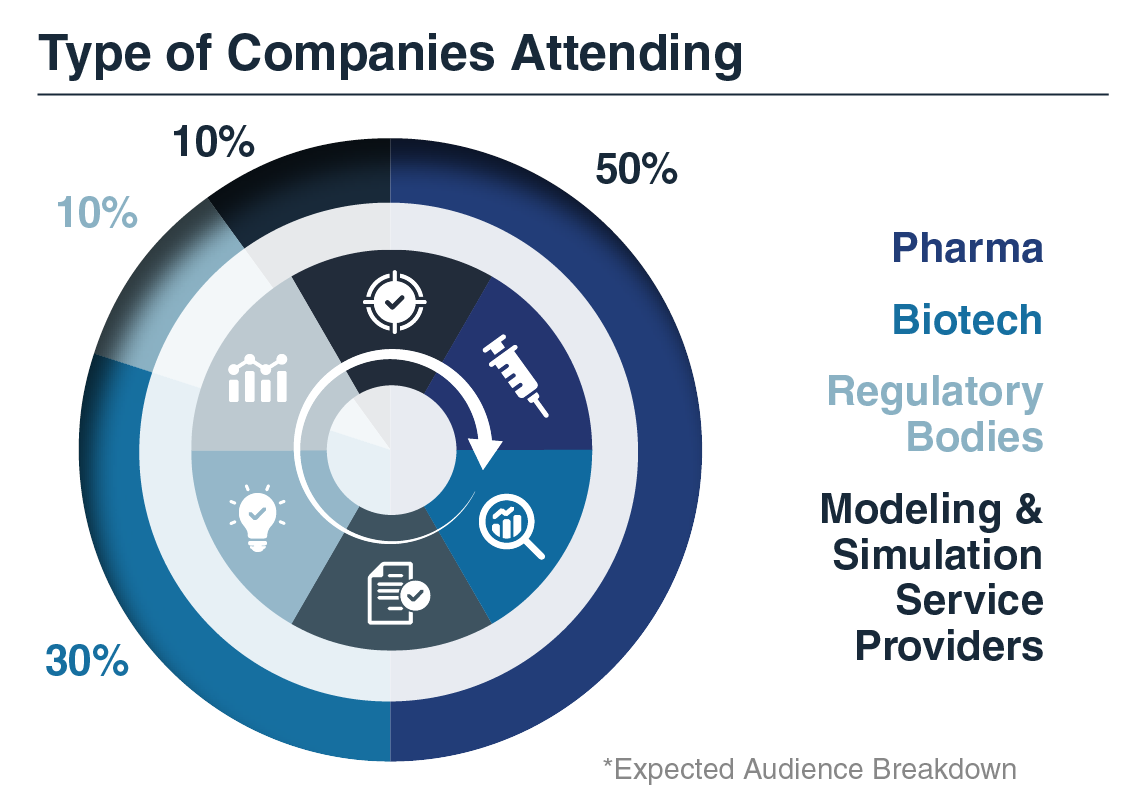
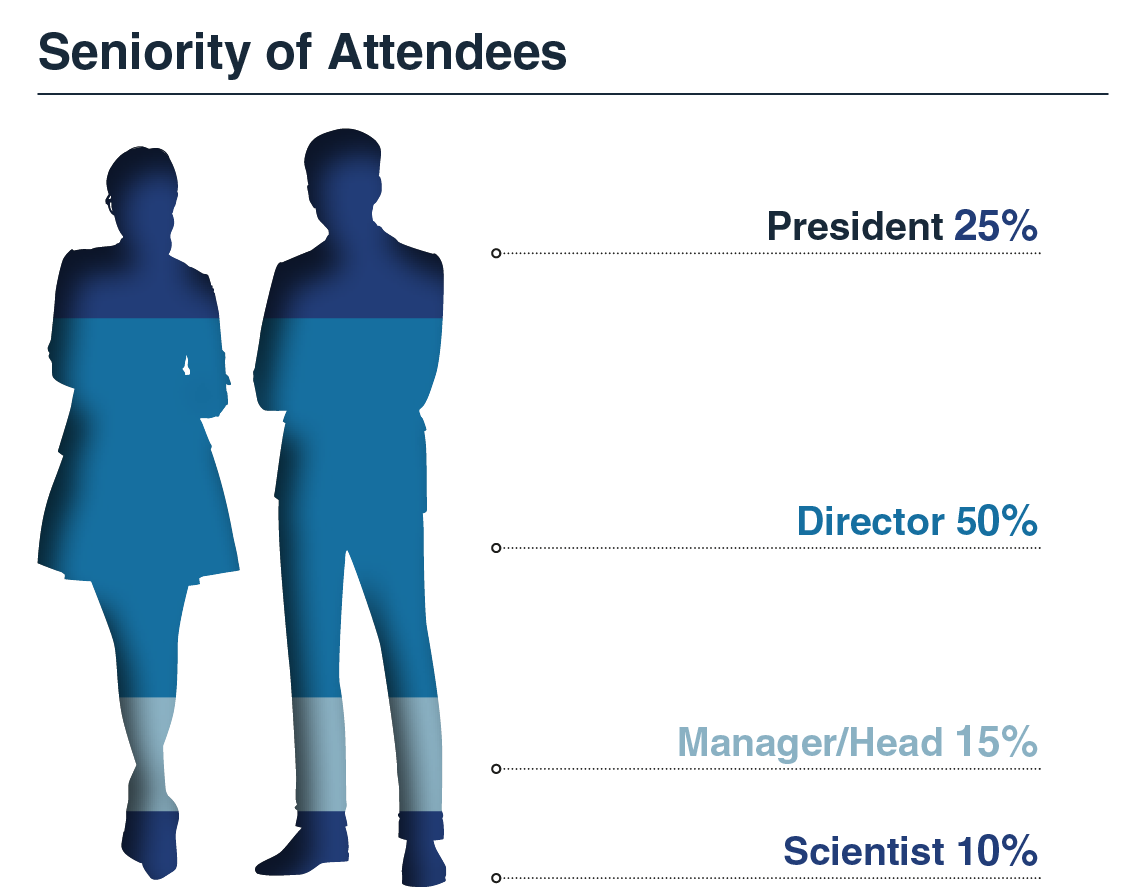
Who Attended?